The dust collector designed specifically for pharmaceutical and containment applications, including the following:
- Tablet presses
- Tablet coating
- Fluid bed drying
- Spray drying
- Blending
- Granulation
- General room ventilation
Gold Series Camtain for Pharmaceutical Manufacturing Applications
- The Gold Series (GS) Camtain units can be used in a variety of pharmaceutical dust collector applications, including tablet presses, coating, fluid bed drying, spray drying, blending, granulation and general room ventilation.
- The GS Camtain is perfect for high-efficiency filtration where recovery of the product is not required.
- Safe-change containment systems are available for both the filter cartridges and discharge system underneath the collector. The cartridge change utilizes the safe-change filter replacement method while the discharge uses continuous liner technology.
- The GS Camtain can also support traditional dust collection for nuisance dusts and fumes that do not require full isolation and containment.
- Camfil APC has containment dust collectors for pharmaceutical applications in North, South, and Central America, Asia and Europe.
Benefits
- In the GS Camtain pharmaceutical dust collector, filter cartridges are sealed via an internal cam bar action, allowing convenient changeout through the safe-change filter replacement system.
- High-entry, cross flow inlet eliminates upward velocities that can hold fine particulates in the filter cartridges, reducing the re-entrainment of the particulate matter.
- Vertically arranged filters shed virtually all the particles versus horizontal filters which allow the particles to build on top of the filter in high dust loading applications.
- High-efficiency filters up to MERV 16 stop 99.99% of the dust at 0.5 microns!
- Specially treated filter media repels fine particulates for a lower pressure drop and longer filter life.
- Gold Cone® cartridge filter design provides 25% more media for long service life. HemiPleat® filter technology ensures uniform pleat spacing with synthetic beads that hold the pleats of the filter cartridge wide open.
The Gold Series contained dust collector is a winner for our pharmaceutical applications. It is way ahead of the curve of anyone in the dust collection industry. The results from the potent compound surrogate test are very positive. Nice work!
– Project Engineer, Major Pharmaceutical Company
Surrogate Tested Dust Collection System for Performance Verification
The Gold Series Camtain pharmaceutical dust collector has been independently surrogate tested for validated performance verification. A major pharmaceutical company's surrogate testing protocol was followed by an independently contracted, AIHA-accredited laboratory (Bureau Veritas) test.
Using 100% milled lactose as the surrogate, we collected over 75 area, personal and swab samples for both the safe-change filter replacement and the continuous liner discharge. The GS Camtain can handle risk-based category 3, 4 and 5 compounds with occupational exposure limits less than 1.0 mcg/m3 for an 8-hour time-weighted average. Full test report data is available upon request. You can download a summary of the surrogate test report now.
Health and Safety Considerations
Two key concerns are critical when handling pharmaceutical dusts: the potent, toxic or allergenic properties of the compound as it relates to personnel exposure and the explosion properties of the compound.
Health/Containment

Continuous liner containment system for dust discharge.
The first issue involves understanding the toxicological properties of the material, reviewing the occupational exposure limit (OEL) and performing a risk-based exposure evaluation to determine the methods for proper control. In most cases, some level of isolation and containment is required due to the fact that the pharmaceutical dust is extremely potent while being captured in a non-production area. Therefore, it cannot be released into the surrounding environment.
In most cases, Camfil APC recommends a HEPA secondary polishing system. With HEPA backup systems and the dust collector, recirculation of the filter air back into the HVAC system is possible. This can significantly reduce energy costs while providing the necessary level of filtration for discharge air required by the EPA.

The cartridge filters in the GS Camtain can be safely replaced by a two-person crew with the safe change filter replacement system.
Safety

Worker safety often requires explosion protection.
The second concern involves deflagration and explosion potential. Control measures such as explosion venting, chemical suppression and isolation systems may be required depending on the physical characteristics of the dust relating to Kst, MIE and the location of the collector.
When explosion vents are required, they must be vented to the outside by either placing the collector outdoors or ducting the vent exhaust a specified distance through the building structure. To prevent outdoor or off-property exposure in the event of a dust explosion of potent compounds, either chemical suppression or containment designs for explosion and isolation valves are preferable to venting. The reason why is because these two protection options do not release materials to the environment.
Camfil APC recommends that an independent PE specifies what explosion protection is required for a given material as it relates to standards in NFPA, ATEX and the major insurance carriers.
Resource Downloads
Here are some informative documents regarding health and safety in industrial dust collection:
Article: Five Ways New Explosion Venting Requirements For Dust Collectors Affect You (PDF)
OSHA Instruction on the Subject of the Combustible Dust National Emphasis Program (PDF)
Summary Table of Surrogate Test Report on the Gold Series Camtain (PDF)
Gold Series Camtain Options
Containment systems and other equipment options designed specifically for the GS Camtain in pharmaceutical applications are available.
Containment Systems
Safe-change containment systems are available for both the filter cartridges and discharge system. The cartridge change utilizes a safe-change filter replacement method while the discharge uses continuous liner technology.
Other Pharma-Specific Options
In addition to the safe-change containment systems above, the GS Camtain has special options just for pharmaceutical applications including:
-
Pharmaceutical upgrade package
- Heavy-duty construction and stiffeners rate housing to 0.44785 bar
- Deflectors to eliminate all internal horizontal ledges
- Closed cell silicone gaskets and caulk at all bolted connections
- HEPA filter on the pressure taps for the pressure differential gauge
-
Explosion vent
- Burst detector
- Vertical plenum
- Up to 1016 mm diameter vent duct
- Weather hood
-
Chemical suppression
-
Chemical isolation
-
Mechanical isolation
Proper Application of the Gold Series Camtain
Camfil APC has an entire team assembled specifically to support the pharmaceutical market. Qualified engineers will work with you to properly apply a Gold Series Camtain pharmaceutical dust collector on your application. Below is a sample of questions/considerations:
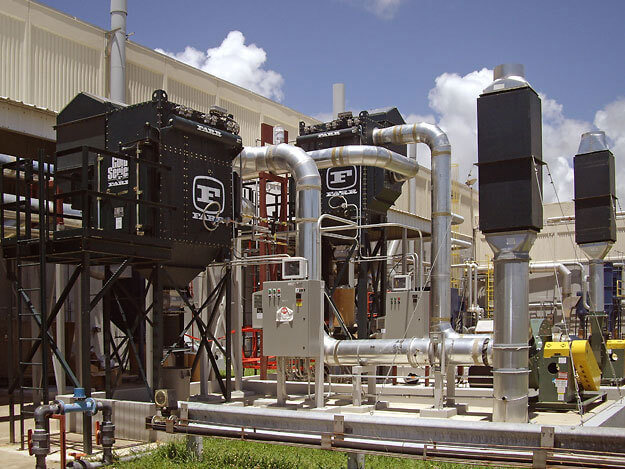
Two Gold Series Camtain CGS6 models on a pharmaceutical application in Guayama, P.R.
- Is the material being collected considered a hazardous material? If so, what is the occupational exposure limit (OEL)?
- What is the source of the dust?
- If the dust is coming off of a process machine, who is the manufacturer?
- What is the air volume that the collector is required to handle?
- What is the estimated dust loading?
- What are the hours of operation?
- What is the required total static pressure the system will need to withstand?
- Will the collector be located indoors or outdoors?
- What is the electrical classification of the room for the collector?
- What are the operating and maximum temperatures?
- What is the relative humidity of the airstream going to the collector?
- What are the Kst value and Pmax of the dust going to the collector?
- Will you be using explosion vents or an explosion suppression system?
- If they want a suppression system, do we need to provide this?
- Do they need isolation for the ductwork? Inlet and Outlet?
- If they are venting, are they venting through a wall (horizontally) or ceiling (vertically)?
- What is the length of the explosion vent ductwork needed?
- Is containment specified? Is it for both the filter change and discharge?
- If the unit is indoors, what are the dimensions of the room?
- Are pre-filters and/or after-filters required? Do they need to be safe-change?
- Do we need to supply the fan (pressure blower)?
- Does the customer currently have dust collectors? Whose? Are they contained?
- What do they like/dislike about collectors/collector manufacturers with which they have experience?

Proper application of the Gold Series Camtain will require a visit to your production facility. Camfil APC has industry professionals dedicated to the pharmaceutical market who can provide a free, on-site demonstration with a trailer-mounted unit.
Users and Specifiers of the Gold Series Camtain
Pharmaceutical End Users
- BOSCH Manesty
- GlaxoSmithKline
- AstraZeneca
- Pfizer
- Abbott
- Biovail
- Bristol-Myers Squibb
- Eli Lilly and Company
- Hikma
- Baxter
- Perrigo
- Novartis
- Tyco Mallinckrodt (Covidien)
- Upsher-Smith
- Merck
- Warner Chilcott
- J & J Ortho-McNeil
- J & J Janssen
- GSK
- Sandoz - Eon Labs
- Aurobindo/Aurolife
- Bayer
- Genzyme
- West Ward (BIRI)
- Amneal
OEMs
- Thomas Engineering
- GEA
- Glatt
- Vector
- O'Hara Technologies
- L.B. Bohle
Engineering Firms
- Jacobs
- Fluor
- CE&IC
- CRB
- IPS
- Stantec
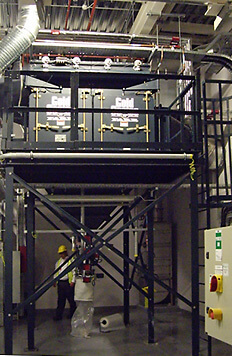
The Gold Series Camtain is now specified by most major pharmaceutical companies.

Insulated Gold Series Camtain units ready for shipment.

Customer inspection of Gold Series Camtain units in Camfil APC's high bay factory.
APC Contacts for Pharmaceutical Applications
Additional Information
David Steil
Pharmaceutical Market Manager
Gilbertsville, PA
Phone: 610-473-9111
Cell: 484-942-5299
Fax: 336-855-9110
Email: david.steil@camfil.com
Horst Hitschler
European Pharmaceutical Market Manager
Tuttlingen, Germany
Phone: +49 (172) 4536894
Email: horst.hitschler@camfil.com


 Australia
Australia 